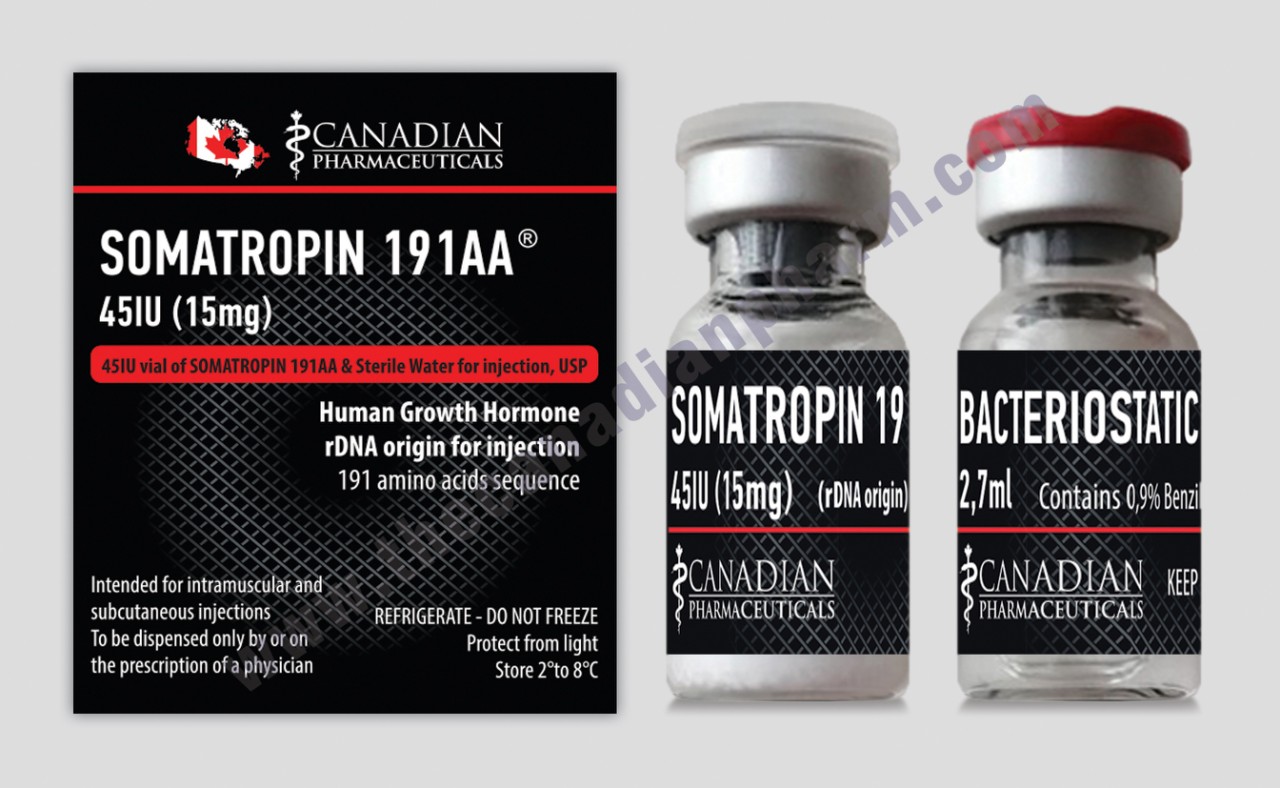
- SOMATROPIN 191AA 45IU (15mg) Canadian Pharmaceuticalas
SOMATROPIN 191AA 45IU on Canadian Pharmaceuticals Company (CPC) is a medicine that contains human growth hormone (also known as somatropin). It is used to treat children aged 2 to 18 years who are failing to grow normally because they do not have enough growth hormone. It is also used in adults with a lack of growth hormone who may have either had growth hormone deficiency as children or developed it later during adulthood.
How is Somatropin 191aa CPC used?
Treatment with Somatropin 191AA (15mg) CPC should be started and monitored by a doctor experienced in diagnosing and treating patients with growth hormone deficiency. The medicine can only be obtained with a prescription.
Somatropin 191aa CPC is available as a powder and solvent which are made into a suspension for injection. Somatropin is injected under the skin once a week. The patient or caregiver can inject Somatropin 191aa CPC themselves, after training by a doctor or a nurse. In children, the recommended dose is 0.5 mg per kilogram body weight, injected once a week. In adults, the recommended dose is 2 mg injected once a week, except for female patients also taking oral oestrogen who should receive 3 mg once a week. The dose may need to be adjusted, depending on the patient’s response to treatment and the side effects the patient experiences.
How does SOMATROPIN 191AA 45IU work?
Growth hormone is a substance secreted by the pituitary gland (a gland located at the base of the brain). It promotes growth during childhood and adolescence, and also affects the way the body handles proteins, fat and carbohydrates. The active substance in Somatropin 191aa CPC is identical to the human growth hormone. It is produced by a method known as ‘recombinant DNA technology’: the hormone is made by yeast cells into which a gene (DNA) has been introduced that makes them able to produce it. Somatropin replaces the natural hormone.
USAGE SCHEME
2,7 ml of solution contains 45 IU somatropin.
12 IU of solution corresponds to 2 IU of somatropin.
18 IU of solution corresponds to 3 IU of somatropin.
24 IU of solution corresponds to 4 IU of somatropin.
30 IU of solution corresponds to 5 IU of somatropin.
36 IU of solution corresponds to 6 IU of somatropin.
42 IU of solution corresponds to 7 IU of somatropin.
48 IU of solution corresponds to 8 IU of somatropin.
54 IU of solution corresponds to 9 IU of somatropin.
60 IU of solution corresponds to10IU of somatropin.
66 IU of solution corresponds to11IU of somatropin.
72 IU of solution corresponds to12IU of somatropin.
What benefits of Somatropin 191aa CPC have been shown in studies?
Somatropin 191aa CPC has been studied in one main study involving 180 children with a lack of growth hormone. The study compared Somatropin 191aa CPC given once a week with another somatropin-containing medicine called Genotropin given once a day. The main measure of effectiveness was the increase in height of patients after one year of treatment. The study showed that Somatropin 191aa CPC was as effective as Genotropin at promoting growth: children receiving Somatropin 191aa CPC grew around 13.7 cm in one year, compared with 12.0 cm in children receiving Genotropin. Somatropin 191aa CPC has also been studied in one main study involving 151 adults with growth hormone deficiency. This study compared Somatropin 191aa CPCwith placebo (a dummy treatment)
and measured the decrease in body fat (which is normally high in adults with growth hormone deficiency) after 6 months of treatment. Adults treated with Somatropin 191aa CPC had an average reduction of body fat of 1 kg while body fat in those treated with placebo increased by 0.5 kg.
What are the risks associated with Somatropin 191aa CPC?
In children, the most common side effects with Somatropin 191aa CPC (which may affect more than 1 in 10 people) are injection site swelling and development of antibodies (proteins that are produced in response to Somatropin CPC). However, these antibodies do not seem to have an effect on the way the medicine works. In adults, the most common side effects (which may affect more than 1 in 10 people) are swelling, mild hyperglycaemia (high blood sugar levels) and headache. For the full list of all side effects reported with Somatropin CPC, see the package leaflet. Somatropin CPC must not be used in patients with an active tumour or an acute lifethreatening illness. For the full list of restrictions, see the package leaflet.
Why is Somatropin CPC approved?
The Agency’s Committee for Medicinal Products for Human Use (CHMP) decided that Somatropin CPC’ benefits are greater than its risks and recommended that it be approved for use in the USA & CANADA. The Committee concluded that Somatropin CPC is as effective in children who fail to grow normally as other somatropin-containing treatments given daily. In adults who lack growth hormone, Somatropin CPC has a modest effect in decreasing body fat. Regarding its safety, the side effects reported with Somatrop

 © Canadian Pharm. All rights reserved
© Canadian Pharm. All rights reserved